 |
Auricular elastic cartilage is a potential source of autologous cells for lining the luminal surfaces of vascular devices such as stents and left ventricular assist devices (LVADs). Cardiovascular devices have been shown to be associated with thromboembolism, bleeding and infection, which are characteristics of poor biocompatibility [2]. To improve the biocompatibility of stents and LVADs whose artificial surfaces will be contacting the blood stream, endothelial cells or genetically engineered smooth muscle cells might be an optimal lining. Endothelial cells secrete, among others factors, chemotactic, growth, and nonthrombogenic factors such as prostacycline and nitric oxide (NO). Nitric oxide, a small gaseous molecule, is known to play a role in vasodilatation, smooth muscle relaxation, programmed cell death, inflammation, the immune system, and in the inhibition of platelet adhesion, aggregation, and activation. There are drawbacks, however, to using either endothelial cells or smooth muscle cells to line cardiovascular devices. Neither cell type is abundantly available nor easily accessible, harvested, or isolated. Moreover, endothelial cells have been observed to slough off easily from artificial surfaces, see [4].
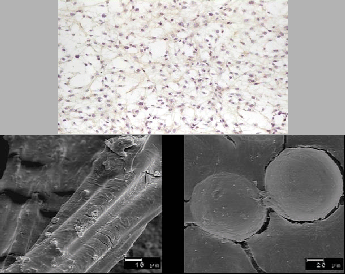
In the search for an alternative cell source, our group has begun investigating the ability of autologous auricular chondrocytes, i.e., ear cartilage cells, to form a strong adherent lining on artificial surfaces of stents. Auricular cartilage harvested from the ear is abundantly available and easily accessible. Auricular chondrocytes have been shown to provide a strong adherent cell lining for left ventricular assist devices because of their ability to synthesize strong adherent extracellular matrix proteins [6]. See Figure 1.1.
 |
 |
To optimize the process of lining of artificial surfaces with auricular cartilage, it is useful to understand how initial cell count, proximity to other cells, and the type of artificial surface affect the rate of cell division and growth of extracellular matrix. In this vein, we designed a mathematical model that explores these issues and provides chondrocyte proliferation and accumulation of the extracellular matrix as a function of time.